The unit periodic trends ionization energy trend WS 3 answers provide a comprehensive exploration of the fascinating patterns and relationships exhibited by ionization energy across the periodic table. This guide delves into the factors that influence ionization energy, including atomic radius, nuclear charge, and electron configuration, and examines the periodic trends within groups and periods.
By understanding these trends, scientists and researchers gain valuable insights into the chemical properties and behavior of elements, enabling them to make informed predictions and guide research and development in fields such as chemistry, physics, and materials science.
Periodic Trends in Ionization Energy
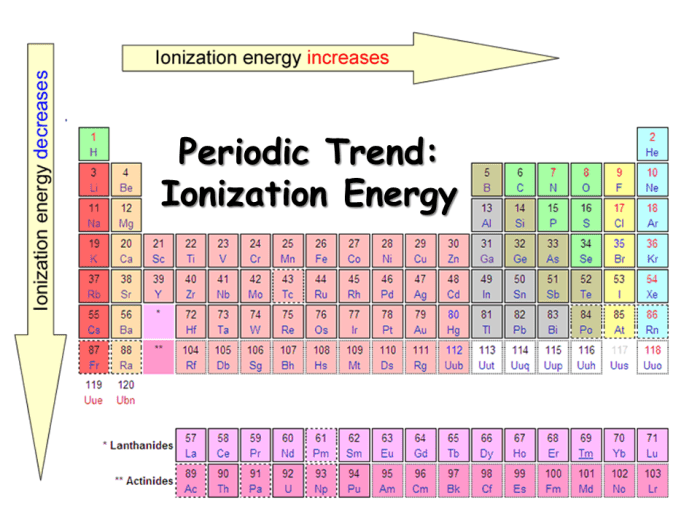
Ionization energy is the energy required to remove an electron from an atom in its gaseous state. It is a fundamental property of elements that influences their chemical reactivity and physical properties. Periodic trends in ionization energy provide insights into the behavior of elements and their placement in the periodic table.
The periodic trends in ionization energy can be summarized as follows:
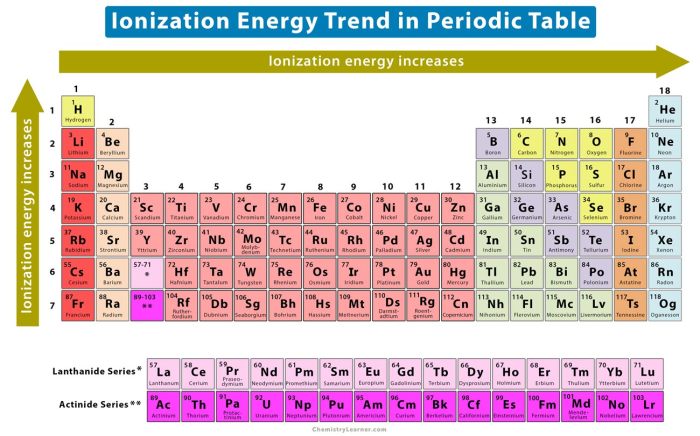
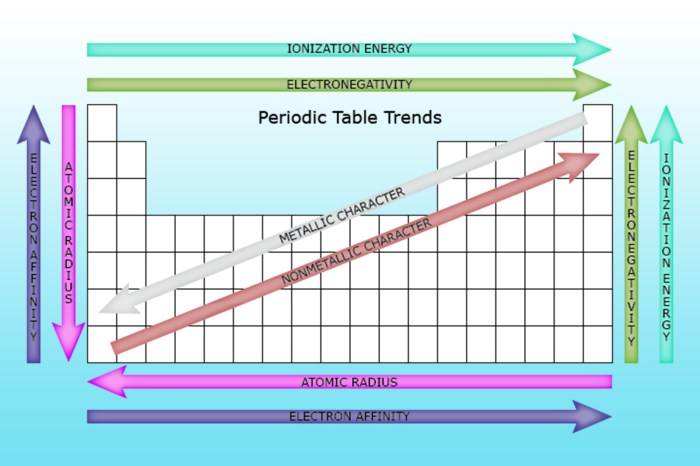
- Ionization energy generally increases from left to right across a period (horizontal row) due to increasing atomic number.
- Ionization energy generally decreases from top to bottom within a group (vertical column) due to increasing atomic radius and shielding effects.
Ionization Energy and Group Trends, Unit periodic trends ionization energy trend ws 3 answers
Within a group, ionization energy decreases from top to bottom. This is because as you move down a group, the atomic radius increases. The larger the atomic radius, the farther the valence electrons are from the nucleus. This makes them less strongly attracted to the nucleus, and therefore easier to remove.
For example, the ionization energy of sodium (Na) is 496 kJ/mol, while the ionization energy of potassium (K), which is below sodium in the same group, is 419 kJ/mol.
Ionization Energy and Period Trends
Within a period, ionization energy generally increases from left to right. This is because as you move from left to right across a period, the atomic number increases. The greater the atomic number, the more protons in the nucleus. This means that the nucleus has a stronger attraction for the electrons, making them more difficult to remove.
For example, the ionization energy of oxygen (O) is 1314 kJ/mol, while the ionization energy of fluorine (F), which is to the right of oxygen in the same period, is 1680 kJ/mol.
Exceptions and Anomalies in Ionization Energy Trends
There are a few exceptions to the general trends in ionization energy. For example, the ionization energy of beryllium (Be) is higher than that of boron (B), even though boron has a higher atomic number. This is because beryllium has a stable electron configuration with a filled 2s orbital, making it less likely to lose an electron.
Applications of Ionization Energy Trends
Ionization energy trends have a wide range of applications in chemistry, physics, and materials science. For example, ionization energy data can be used to:
- Predict the chemical reactivity of elements
- Design new materials with desired properties
- Understand the behavior of atoms in chemical reactions
Top FAQs: Unit Periodic Trends Ionization Energy Trend Ws 3 Answers
What is ionization energy?
Ionization energy is the energy required to remove an electron from an atom or ion in its gaseous state.
How does ionization energy vary across the periodic table?
Ionization energy generally increases from left to right across a period and decreases from top to bottom within a group.
What factors influence ionization energy?
Factors influencing ionization energy include atomic radius, nuclear charge, and electron configuration.