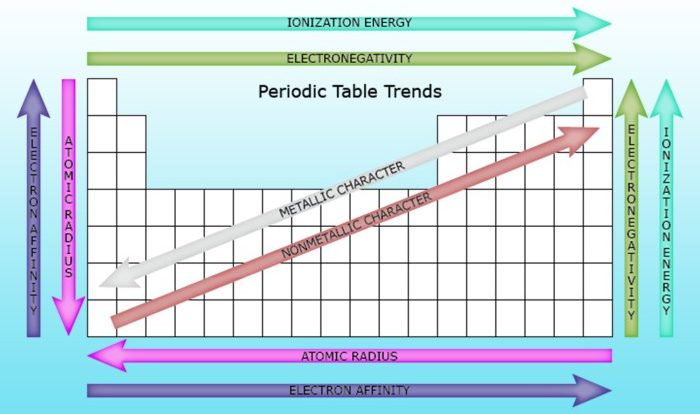
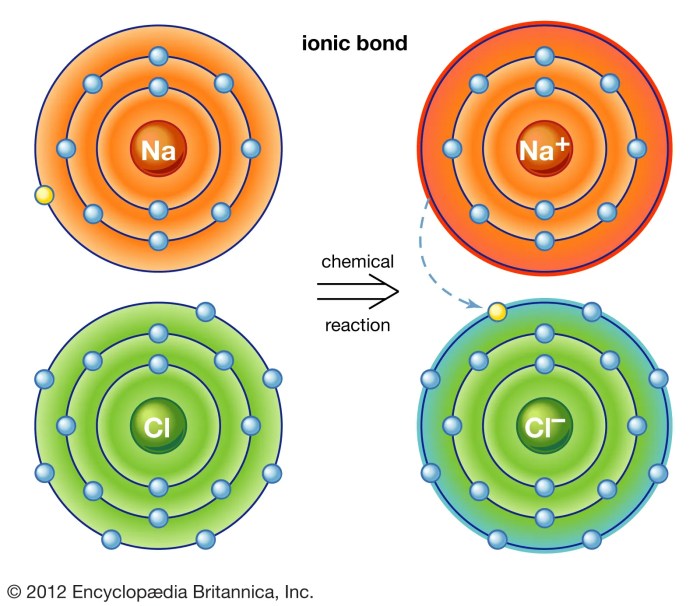
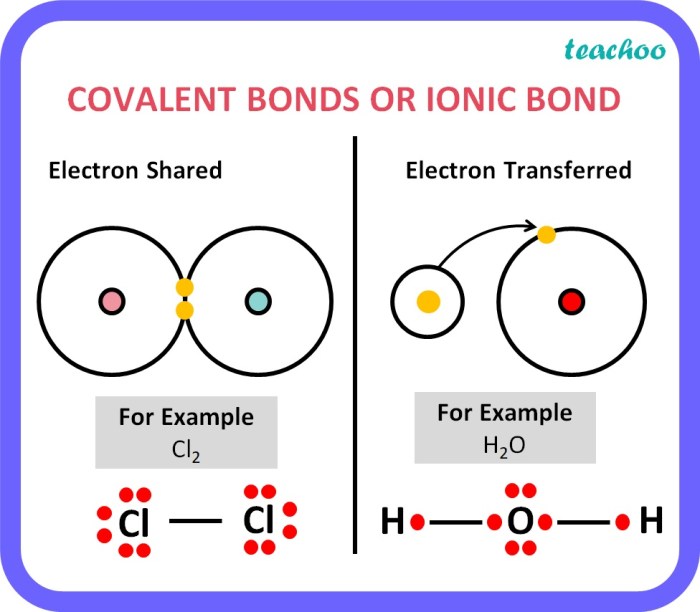
Delving into the realm of ionic bonds and ionic compounds 7.2, this exploration unravels the captivating intricacies of these fundamental chemical entities. Ionic bonds, the electrostatic forces between positively charged cations and negatively charged anions, play a pivotal role in shaping the properties and applications of ionic compounds.
Ionic compounds exhibit a unique array of physical and chemical characteristics, ranging from their high melting and boiling points to their remarkable solubility in polar solvents. These properties stem from the strong electrostatic interactions between the constituent ions, which are held together in a highly organized crystal lattice.
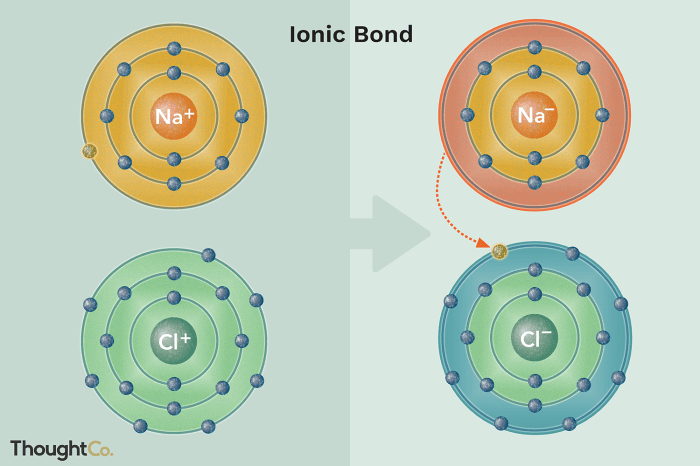
Ionic Bond Formation
Ionic bond formation occurs when one atom transfers one or more electrons to another atom, resulting in the formation of positively and negatively charged ions. The positive ion is called a cation, while the negative ion is called an anion.
The electrostatic attraction between the oppositely charged ions holds the ionic compound together.
Factors Affecting Ionic Bond Strength
- Charge of the ions: The greater the charge of the ions, the stronger the ionic bond.
- Size of the ions: Smaller ions have a stronger attraction for electrons, leading to stronger ionic bonds.
- Lattice energy: The energy required to separate ions in a crystal lattice is a measure of ionic bond strength.
Properties of Ionic Compounds: Ionic Bonds And Ionic Compounds 7.2
Ionic compounds generally have high melting and boiling points, indicating strong interionic forces. They are typically hard and brittle due to the rigid arrangement of ions in the crystal lattice. Ionic compounds are good conductors of electricity when dissolved in water or melted, as the ions can move freely in solution or in the molten state.
Relationship between Ionic Bond Strength and Compound Properties
Stronger ionic bonds result in higher melting and boiling points, increased hardness, and decreased solubility. This is because stronger bonds require more energy to break, leading to higher melting and boiling points, and a more rigid crystal structure that makes the compound harder.
Lattice Energy
Lattice energy is the energy required to separate all the ions in a crystal lattice into individual gaseous ions. It is a measure of the strength of the ionic bond in the compound.
Factors Affecting Lattice Energy, Ionic bonds and ionic compounds 7.2
- Charge of the ions: Higher charge ions result in higher lattice energy.
- Size of the ions: Smaller ions have higher lattice energy.
- Crystal structure: Different crystal structures can affect the lattice energy.
Hydration Energy
Hydration energy is the energy released when ions in a crystal lattice are surrounded by water molecules. It is a measure of the strength of the interaction between the ions and water molecules.
Factors Affecting Hydration Energy
- Charge of the ion: Higher charge ions have higher hydration energy.
- Size of the ion: Smaller ions have higher hydration energy.
- Polarizability of the ion: More polarizable ions have higher hydration energy.
Solubility of Ionic Compounds
The solubility of ionic compounds in water depends on the balance between lattice energy and hydration energy. Compounds with high lattice energy and low hydration energy are less soluble, while compounds with low lattice energy and high hydration energy are more soluble.
Applications of Solubility Rules
Solubility rules can be used to predict the solubility of ionic compounds based on the identity of the cation and anion. These rules can be useful in various applications, such as qualitative analysis and the design of chemical processes.
Applications of Ionic Compounds
Ionic compounds have a wide range of applications in everyday life. Some common examples include:
- Sodium chloride (table salt): Used as a seasoning and preservative.
- Potassium chloride: Used as a fertilizer and in medicine.
- Calcium carbonate (limestone): Used in construction and as an antacid.
- Sodium bicarbonate (baking soda): Used as a leavening agent and in cleaning products.
- Potassium nitrate (saltpeter): Used in fertilizers and explosives.
Question Bank
What are the key factors affecting ionic bond strength?
Ionic bond strength is primarily determined by the charges of the ions involved and the distance between them.
How does lattice energy influence the properties of ionic compounds?
Lattice energy is directly proportional to the strength of the electrostatic interactions between ions. Higher lattice energy leads to higher melting and boiling points, as well as lower solubility.
What applications do ionic compounds have in everyday life?
Ionic compounds are essential components in various industries, including the production of fertilizers, glass, ceramics, and pharmaceuticals.