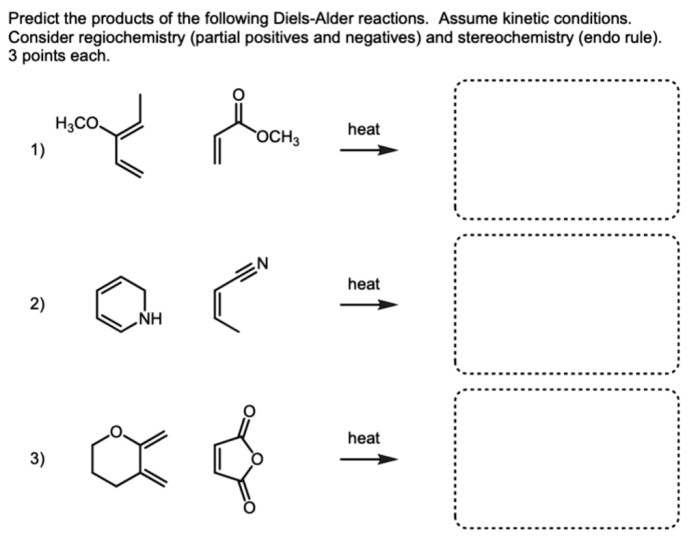
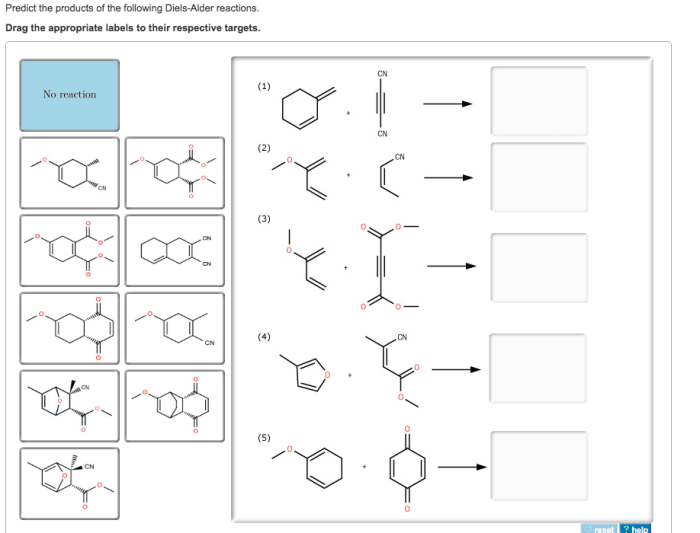
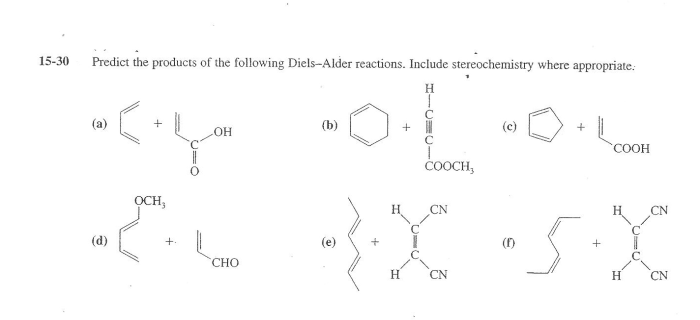
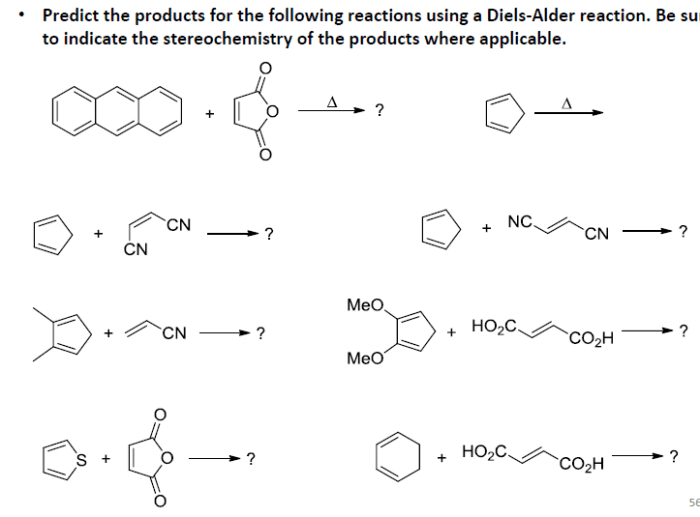
Predict the products of the following diels alder reactions – Delving into the realm of Diels-Alder reactions, this comprehensive guide unveils the intricate mechanisms and predictive strategies employed to decipher the products of these versatile cycloadditions. With a focus on regioselectivity and stereoselectivity, we embark on a journey to unravel the factors that govern the orientation and reactivity of dienes and dienophiles, empowering chemists to harness the synthetic potential of these powerful reactions.
As we delve deeper into the Diels-Alder reaction mechanism, we will explore the cycloaddition process in detail, unraveling the key steps and illustrating them with chemical equations. By understanding the electronic interactions and frontier molecular orbital theory, we gain insights into the regio- and stereoselective outcomes of these reactions.
Diels-Alder Reaction Mechanism
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile. The reaction proceeds through a concerted mechanism, meaning that all of the bonds are formed or broken at the same time. The first step of the reaction is the formation of a π-complex between the diene and the dienophile.
This π-complex is then converted to a σ-complex, which is the final product of the reaction.
The Diels-Alder reaction is a powerful tool for the synthesis of cyclic compounds. It can be used to construct a wide variety of ring sizes and structures. The reaction is also highly regio- and stereoselective, meaning that the products of the reaction can be controlled by the choice of diene and dienophile.
Predicting Products of Diels-Alder Reactions, Predict the products of the following diels alder reactions
The regioselectivity and stereoselectivity of Diels-Alder reactions can be predicted using Frontier Molecular Orbital (FMO) theory. FMO theory states that the HOMO (highest occupied molecular orbital) of the diene will interact with the LUMO (lowest unoccupied molecular orbital) of the dienophile.
The orientation of the diene and the dienophile will be such that the HOMO-LUMO interaction is maximized.
The regioselectivity of the Diels-Alder reaction can also be controlled by the use of substituents on the diene and the dienophile. Electron-withdrawing substituents on the diene will make the diene less reactive, while electron-donating substituents on the dienophile will make the dienophile more reactive.
This can be used to control the regioselectivity of the reaction.
Examples of Diels-Alder Reactions
The Diels-Alder reaction is a versatile reaction that can be used to synthesize a wide variety of cyclic compounds. Some examples of Diels-Alder reactions include:
- The synthesis of cyclohexene from 1,3-butadiene and ethylene
- The synthesis of cyclopentadiene from 1,3-cyclopentadiene and ethylene
- The synthesis of indene from indene and ethylene
Applications of Diels-Alder Reactions
The Diels-Alder reaction is a powerful tool for the synthesis of cyclic compounds. It is used in the synthesis of a wide variety of natural products and pharmaceuticals. Some examples of the applications of Diels-Alder reactions include:
- The synthesis of the antibiotic penicillin
- The synthesis of the anticancer drug Taxol
- The synthesis of the vitamin D precursor cholecalciferol
FAQ Corner: Predict The Products Of The Following Diels Alder Reactions
What is the Diels-Alder reaction?
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a cyclohexene ring.
How can I predict the regioselectivity of a Diels-Alder reaction?
Regioselectivity can be predicted using frontier molecular orbital (FMO) theory, which considers the interactions between the highest occupied molecular orbital (HOMO) of the diene and the lowest unoccupied molecular orbital (LUMO) of the dienophile.
What factors influence the stereoselectivity of a Diels-Alder reaction?
Stereoselectivity can be influenced by substituents on the diene and dienophile, as well as by the reaction conditions.