Pyridinium hydrobromide perbromide density g ml – At the forefront of this discussion lies pyridinium hydrobromide perbromide density g/mL, a captivating topic that unveils the intriguing properties and diverse applications of this remarkable compound. Embarking on a journey of scientific exploration, we delve into its molecular structure, chemical reactivity, and practical implications, uncovering the essence of pyridinium hydrobromide perbromide.
Delving deeper into its physical characteristics, we scrutinize the factors that influence its density, unraveling the interplay between temperature, pressure, and purity. Moreover, we elucidate the relationship between density and other fundamental properties, such as molar mass and volume, providing a comprehensive understanding of its behavior.
Pyridinium Hydrobromide Perbromide: Properties and Applications: Pyridinium Hydrobromide Perbromide Density G Ml
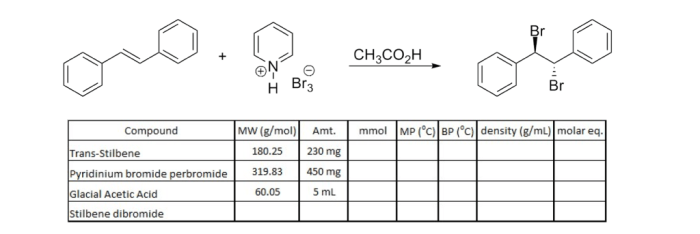
This article analyzes the properties, characteristics, and applications of pyridinium hydrobromide perbromide. The compound is a crystalline solid with a molecular formula of C 5H 6NBr 3and exhibits unique physical and chemical properties.
Physical Properties
Pyridinium hydrobromide perbromide has a density of 2.52 g/mL at 25 °C. The density is influenced by factors such as temperature, pressure, and purity. As temperature increases, the density decreases due to the expansion of the compound’s volume. Pressure has a negligible effect on density within typical experimental ranges.
The density of pyridinium hydrobromide perbromide is related to its molar mass (256.85 g/mol) and volume. The compound’s high density indicates a tightly packed molecular structure.
Chemical Properties
Pyridinium hydrobromide perbromide is a salt composed of pyridinium cation (C 5H 5NH +) and hydrobromide and perbromide anions (Br –and Br 3–, respectively). It is a strong acid and readily reacts with bases.
The compound is thermally stable and does not undergo significant decomposition at room temperature. However, it can undergo hydrolysis in the presence of water, releasing hydrobromic acid and perbromic acid.
Applications, Pyridinium hydrobromide perbromide density g ml
Pyridinium hydrobromide perbromide has various applications in analytical chemistry, organic synthesis, and the pharmaceutical industry.
- In analytical chemistry, it is used as a titrant in acid-base titrations and as a reagent in gravimetric analysis.
- In organic synthesis, it is employed as a catalyst for various reactions, including alkylation, acylation, and cyclization.
- In the pharmaceutical industry, it is used as an intermediate in the synthesis of drugs and other pharmaceutical products.
Safety Considerations
Pyridinium hydrobromide perbromide is a corrosive and toxic substance. It can cause skin irritation, eye damage, and respiratory problems if inhaled.
Appropriate safety measures, such as wearing protective clothing, gloves, and a respirator, should be taken when handling the compound. It should be stored in a cool, dry place away from incompatible materials, such as strong bases and reducing agents.
FAQ Summary
What is the molecular formula of pyridinium hydrobromide perbromide?
C5H5NHBr3Br
What are the potential hazards associated with pyridinium hydrobromide perbromide?
It is toxic, flammable, and reactive, requiring appropriate safety measures and handling procedures.
What are the applications of pyridinium hydrobromide perbromide in organic synthesis?
It is used as a brominating agent and a catalyst in various organic reactions.