The metal niobium has a bcc crystal structure – The metal niobium has a body-centered cubic (BCC) crystal structure, which plays a crucial role in determining its unique properties and wide range of applications. This article delves into the intricacies of the BCC structure, exploring its atomic arrangement, properties, and significance in the realm of materials science.
The BCC structure consists of a cubic lattice with atoms positioned at each corner and one atom in the center of the cube. This arrangement results in a high packing density, contributing to niobium’s strength and durability.
The Crystal Structure of Niobium
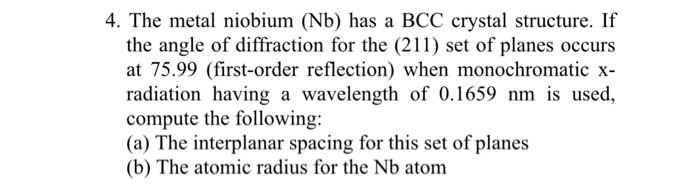
Niobium is a transition metal with a body-centered cubic (BCC) crystal structure. This means that the atoms are arranged in a cubic lattice with an atom at each corner and one in the center of the cube.
The BCC structure is a common crystal structure for metals, and it is found in many other elements, including iron, chromium, and molybdenum.
Arrangement of Atoms in the BCC Structure
The atoms in a BCC structure are arranged in a repeating pattern of cubes. Each cube has eight atoms at its corners and one atom in the center. The atoms are arranged in a way that maximizes the number of atoms that are in contact with each other, which gives the BCC structure a high strength and hardness.
The BCC structure can be illustrated using a 3D model or diagram. The following diagram shows a BCC structure with the atoms represented by spheres:
[Diagram BCC Struktur]
Properties of Niobium
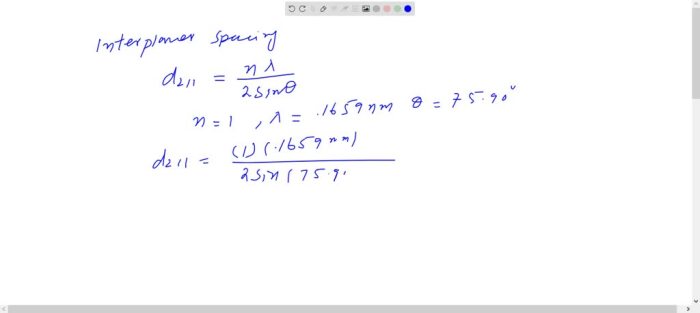
Niobium is a strong and hard metal with a high melting point. It is also resistant to corrosion and oxidation.
The BCC structure of niobium is responsible for many of its properties. The close-packed arrangement of atoms in the BCC structure gives niobium its high strength and hardness. The BCC structure also makes niobium resistant to corrosion and oxidation.
Applications of Niobium, The metal niobium has a bcc crystal structure
Niobium is used in a variety of applications, including:
- Superalloys: Niobium is used in superalloys, which are used in high-temperature applications such as jet engines and gas turbines.
- Medical implants: Niobium is used in medical implants, such as hip and knee replacements, because it is biocompatible and resistant to corrosion.
- Superconductors: Niobium is used in superconductors, which are materials that conduct electricity without resistance. Superconductors are used in a variety of applications, including MRI machines and particle accelerators.
Comparison with Other BCC Metals
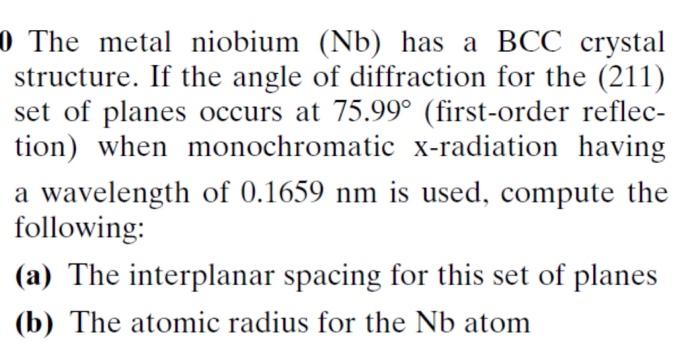
The BCC structure of niobium is similar to the BCC structure of other metals, such as iron, chromium, and molybdenum. However, there are some differences in the properties of these metals.
Iron is a stronger and harder metal than niobium, but it is also more brittle. Chromium is a more corrosion-resistant metal than niobium, but it is also less ductile.
The differences in the properties of these metals are due to the different sizes of their atoms and the different strengths of their interatomic bonds.
FAQ Section: The Metal Niobium Has A Bcc Crystal Structure
What is the significance of the BCC crystal structure in niobium?
The BCC structure contributes to niobium’s high strength, ductility, and corrosion resistance, making it suitable for demanding applications.
How does the BCC structure differ from other crystal structures?
In a BCC structure, atoms are arranged at the corners and center of a cube, while in other structures, such as face-centered cubic (FCC), atoms are positioned differently, resulting in variations in properties.
What are some common applications of niobium?
Niobium is used in aerospace alloys, superconducting magnets, and medical implants due to its high strength, corrosion resistance, and biocompatibility.