Identify arrows pointing to bonding electrons. – In the realm of chemistry, understanding the intricacies of bonding electrons is paramount. This guide delves into the concept of bonding electrons, exploring their role in forming chemical bonds and the various methods used to represent them. By identifying arrows pointing to bonding electrons, we gain a deeper insight into the electronic structure and behavior of molecules.
Delving further, we will examine the different types of arrows employed to indicate bonding electrons, including single arrows, double arrows, and curved arrows. Each arrow type carries a specific meaning, providing valuable information about the nature of the bond. Through practical examples and step-by-step instructions, we will demonstrate how to use these arrows to represent the movement of bonding electrons, a crucial aspect in understanding chemical reactions.
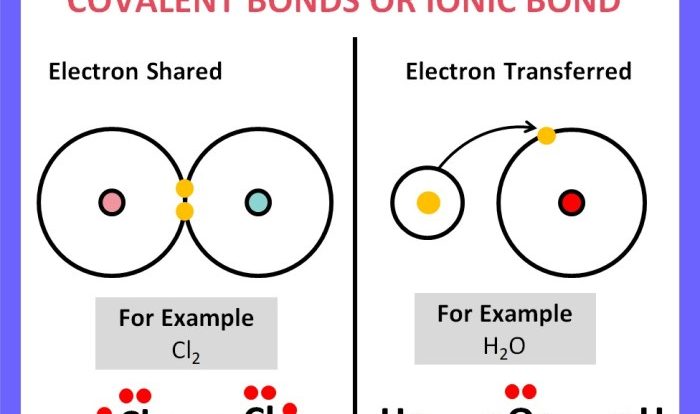
Identify Arrows Pointing to Bonding Electrons: Identify Arrows Pointing To Bonding Electrons.

Identifying arrows pointing to bonding electrons is crucial in chemistry, as they provide valuable information about the structure and behavior of molecules. This article will discuss the different types of arrows used to indicate bonding electrons, their meanings, and how to use them effectively.
Different Types of Bonding Electrons
Bonding electrons are the electrons that participate in the formation of chemical bonds between atoms. They can be classified into two main types:
- Sigma (σ) electrons:These electrons are located directly along the internuclear axis, forming a head-to-head overlap between atomic orbitals.
- Pi (π) electrons:These electrons are located above and below the internuclear axis, forming a lateral overlap between atomic orbitals.
Representing Bonding Electrons, Identify arrows pointing to bonding electrons.
There are two main methods for representing bonding electrons:
- Lewis dot structures:These structures show the valence electrons of atoms as dots around the atomic symbols. Bonding electrons are represented as lines connecting the atomic symbols.
- Molecular orbital diagrams:These diagrams show the molecular orbitals of a molecule and the distribution of electrons within them. Bonding electrons are represented as arrows pointing from the lower-energy orbital to the higher-energy orbital.
Arrows Indicating Bonding Electrons
Arrows can be used to indicate bonding electrons in various ways:
- Single arrows:These arrows indicate a single bond between two atoms. The arrow points from the atom donating the electron to the atom receiving the electron.
- Double arrows:These arrows indicate a double bond between two atoms. The two arrows represent the two pairs of electrons involved in the bond.
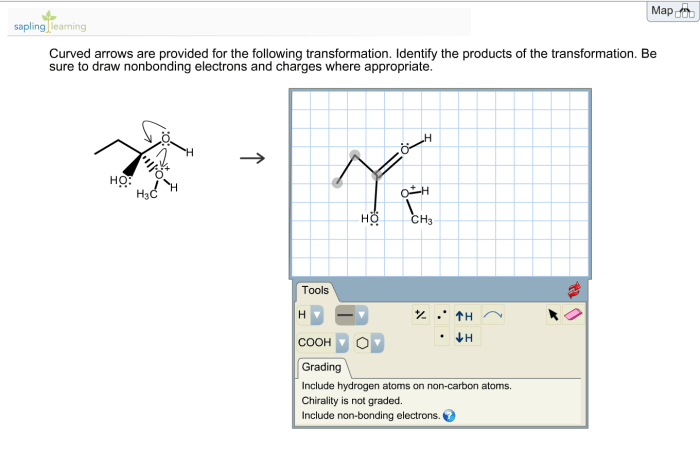
- Curved arrows:These arrows are used to indicate the movement of electrons in chemical reactions. The arrowhead points in the direction of electron flow.
Using Arrows to Indicate Electron Movement
Arrows can be used to indicate the movement of bonding electrons in chemical reactions:
- Identify the atoms involved in the reaction and the type of bond that is being formed or broken.
- Draw an arrow from the atom donating the electron to the atom receiving the electron.
- If necessary, use curved arrows to show the movement of electrons.
Table of Arrows Indicating Bonding Electrons
| Type of Arrow | Meaning | Example |
|---|---|---|
| Single arrow | Single bond | H->Cl |
| Double arrow | Double bond | O=C=O |
| Curved arrow | Electron movement | CH3->CH2 |
Identifying Bonding Electrons
Bonding electrons can be identified in various ways:
- By their location:Bonding electrons are located in the region of space between bonded atoms.
- By their energy:Bonding electrons are typically lower in energy than non-bonding electrons.
- By their spin:Bonding electrons have opposite spins, forming a singlet state.
Questions and Answers
What is the purpose of using arrows to indicate bonding electrons?
Arrows are used to represent the movement of bonding electrons during chemical reactions, providing a visual representation of the electron flow.
How do I identify single and double bonds using arrows?
Single arrows represent single bonds, while double arrows represent double bonds. The number of arrows corresponds to the bond order.
What is the significance of curved arrows in representing electron movement?
Curved arrows indicate the direction of electron flow during chemical reactions, showing the transfer or sharing of electrons between atoms.