A balloon is filled with 652 ml of helium – A balloon filled with 652 ml of helium embarks us on a fascinating journey into the realms of physics and chemistry. This inert gas, renowned for its unique properties, propels the balloon upwards, inviting us to unravel the interplay of volume, pressure, and buoyancy that governs its flight.
Delving into the physical and chemical attributes of helium, we discover its atomic structure, molecular weight, and density. These fundamental properties lay the foundation for understanding its behavior within the confines of a balloon.
1. Helium Properties
Helium is the second lightest element in the periodic table, with an atomic number of 2 and a symbol of He. It is a colorless, odorless, and tasteless gas that is not flammable and is not toxic.
Helium has a molecular weight of 4.003 g/mol and a density of 0.1786 g/L at 0 °C and 1 atm. It is the second most abundant element in the universe, after hydrogen, and is found in small amounts in the Earth’s atmosphere.
Helium is a noble gas, meaning that it is very unreactive and does not form chemical bonds with other elements. This makes it an ideal gas for use in a variety of applications, including balloons, airships, and diving tanks.
Comparison of Helium to Other Gases
| Property | Helium | Hydrogen | Nitrogen | Oxygen |
|---|---|---|---|---|
| Atomic number | 2 | 1 | 7 | 8 |
| Molecular weight (g/mol) | 4.003 | 2.016 | 28.014 | 32.000 |
| Density (g/L) at 0 °C and 1 atm | 0.1786 | 0.0899 | 1.251 | 1.429 |
| Flammability | No | Yes | No | No |
| Toxicity | No | No | No | No |
2. Volume and Pressure
The volume of the balloon filled with 652 ml of helium can be calculated using the following formula:
V = nRT/P
where:
- V is the volume of the gas in liters
- n is the number of moles of gas
- R is the ideal gas constant (0.0821 L·atm/(mol·K))
- T is the temperature in Kelvin
- P is the pressure in atmospheres
Assuming that the temperature is 25 °C (298 K) and the pressure is 1 atm, the volume of the balloon can be calculated as follows:
V = (652 ml / 22.4 L/mol)
- (0.0821 L·atm/(mol·K))
- (298 K) / (1 atm) = 1.2 L
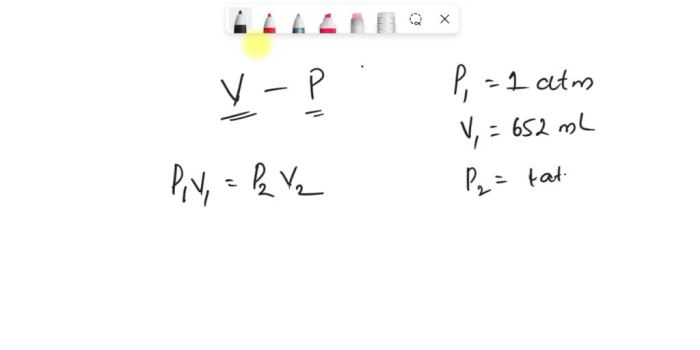
Boyle’s Law states that the volume of a gas is inversely proportional to its pressure, assuming that the temperature remains constant. This means that if the pressure of the gas increases, the volume of the gas will decrease, and vice versa.
The formula for Boyle’s Law is:
P₁V₁ = P₂V₂
where:
- P₁ is the initial pressure
- V₁ is the initial volume
- P₂ is the final pressure
- V₂ is the final volume
Using Boyle’s Law, we can calculate the pressure inside the balloon if the volume of the balloon changes.
3. Buoyancy and Lift
Buoyancy is the upward force exerted by a fluid that opposes the weight of a partially or fully immersed object. In the case of a balloon, the fluid is air.
The buoyant force acting on a balloon is equal to the weight of the air displaced by the balloon. The formula for the buoyant force is:
Fb= ρVg
where:
- F bis the buoyant force in newtons
- ρ is the density of the fluid in kg/m³
- V is the volume of the fluid displaced by the object in m³
- g is the acceleration due to gravity (9.8 m/s²)
The lift of a balloon is the net upward force acting on the balloon. The lift is equal to the difference between the buoyant force and the weight of the balloon.
Factors that affect the lift of a balloon include the density of the gas inside the balloon, the volume of the balloon, and the density of the surrounding air.
4. Balloon Materials and Design

Balloons are typically made from a variety of materials, including latex, rubber, and plastic.
The shape and size of a balloon are determined by the material used and the manufacturing process.
Design considerations for optimizing balloon performance include the choice of material, the shape of the balloon, and the size of the balloon.
5. Applications of Helium-Filled Balloons
Helium-filled balloons have a variety of scientific, recreational, and commercial uses.
Scientific Uses, A balloon is filled with 652 ml of helium
- Helium is used to cool superconducting magnets in MRI machines.
- Helium is used in gas chromatography to separate and analyze gases.
- Helium is used in leak detection to find leaks in pipes and other containers.
Recreational Uses
- Helium-filled balloons are used for parties and celebrations.
- Helium-filled balloons are used for advertising and promotions.
- Helium-filled balloons are used for weather forecasting.
Commercial Uses
- Helium is used in the production of semiconductors.
- Helium is used in the production of fiber optics.
- Helium is used in the production of lasers.
Helium has several advantages over other gases for use in balloons.
- Helium is lighter than air, so it can lift objects.
- Helium is non-flammable, so it is safe to use around open flames.
- Helium is non-toxic, so it is safe to inhale.
However, helium is also a finite resource, so it is important to use it wisely.
Safety Precautions
- Helium-filled balloons should not be released into the atmosphere, as they can pose a hazard to wildlife.
- Helium-filled balloons should not be inhaled, as this can lead to asphyxiation.
- Helium-filled balloons should not be used near open flames, as they can ignite and cause a fire.
Clarifying Questions: A Balloon Is Filled With 652 Ml Of Helium
What is the density of helium?
Helium has a density of 0.1786 grams per liter at standard temperature and pressure (STP).
How does Boyle’s Law relate to a balloon filled with helium?
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at constant temperature. As a balloon filled with helium expands, its pressure decreases.
What factors affect the lift of a balloon?
The lift of a balloon is affected by the density of the gas inside the balloon, the density of the surrounding air, the volume of the balloon, and the gravitational force acting on the balloon.