Embark on a captivating exploration of the solid liquid gas venn diagram, a visual masterpiece that unveils the intriguing similarities and differences between the three fundamental states of matter: solid, liquid, and gas. Dive into the fascinating world of matter and discover the secrets behind its diverse behaviors.
From the molecular structure of each state to the energy changes associated with phase transitions, this comprehensive guide delves into the intricacies of matter’s transformations. Prepare to be amazed as we unravel the implications of the Venn diagram for understanding the behavior of matter in our world.
Properties of Solids, Liquids, and Gases
Solids, liquids, and gases are the three fundamental states of matter. Each state exhibits distinct physical properties and molecular arrangements. Understanding these properties is crucial for comprehending various phenomena in chemistry, physics, and everyday life.
Defining Characteristics
Solids possess a fixed shape and volume, meaning they maintain their shape and occupy a specific amount of space. The molecules in solids are tightly packed and arranged in a regular, crystalline structure. This rigid structure gives solids their characteristic strength and rigidity.Liquids,
on the other hand, have a fixed volume but no definite shape. They assume the shape of their container and flow easily. The molecules in liquids are loosely packed and can move more freely than in solids. This fluidity allows liquids to adapt to different shapes and flow through narrow openings.Gases
have neither a fixed shape nor a fixed volume. They expand to fill the entire volume of their container and have very low density. The molecules in gases are widely spaced and move rapidly in all directions. This high kinetic energy gives gases their ability to expand and compress easily.
Examples
Some common examples of solids include ice, rock, and metal. Examples of liquids include water, oil, and milk. Examples of gases include air, hydrogen, and helium.
Molecular Structure and Arrangement
The molecular structure and arrangement of solids, liquids, and gases vary significantly. In solids, the molecules are tightly packed in a regular, crystalline lattice. This arrangement results in a strong, rigid structure. In liquids, the molecules are loosely packed and can move more freely, forming a more fluid structure.
In gases, the molecules are widely spaced and move rapidly in all directions, resulting in a highly expansive and compressible structure.
Phase Transitions
Phase transitions are physical changes in which a substance changes from one state of matter to another. The three main phase transitions are melting, freezing, and vaporization.
Melting
Melting is the phase transition from a solid to a liquid. When a solid melts, the particles gain enough energy to overcome the attractive forces holding them in a fixed position. As a result, the particles become more mobile and the substance changes from a solid to a liquid.
The energy required to melt a substance is called the heat of fusion. The heat of fusion is the amount of energy required to change one mole of a solid to a liquid at its melting point.
Freezing
Freezing is the phase transition from a liquid to a solid. When a liquid freezes, the particles lose energy and slow down. As the particles slow down, they become more ordered and the substance changes from a liquid to a solid.
The energy released when a liquid freezes is called the heat of crystallization. The heat of crystallization is the amount of energy released when one mole of a liquid changes to a solid at its freezing point.
Vaporization
Vaporization is the phase transition from a liquid to a gas. When a liquid vaporizes, the particles gain enough energy to overcome the attractive forces holding them together. As a result, the particles become much more mobile and the substance changes from a liquid to a gas.
The energy required to vaporize a substance is called the heat of vaporization. The heat of vaporization is the amount of energy required to change one mole of a liquid to a gas at its boiling point.
Examples of Phase Transitions in Everyday Life
Phase transitions are all around us. Here are a few examples:
- Water freezing into ice
- Ice melting into water
- Water boiling into steam
- Steam condensing into water
- Dry ice sublimating into carbon dioxide gas
Venn Diagram: Solid Liquid Gas Venn Diagram
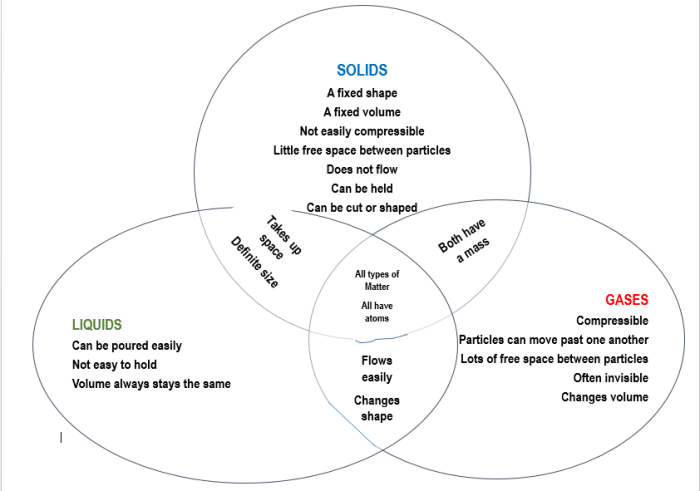
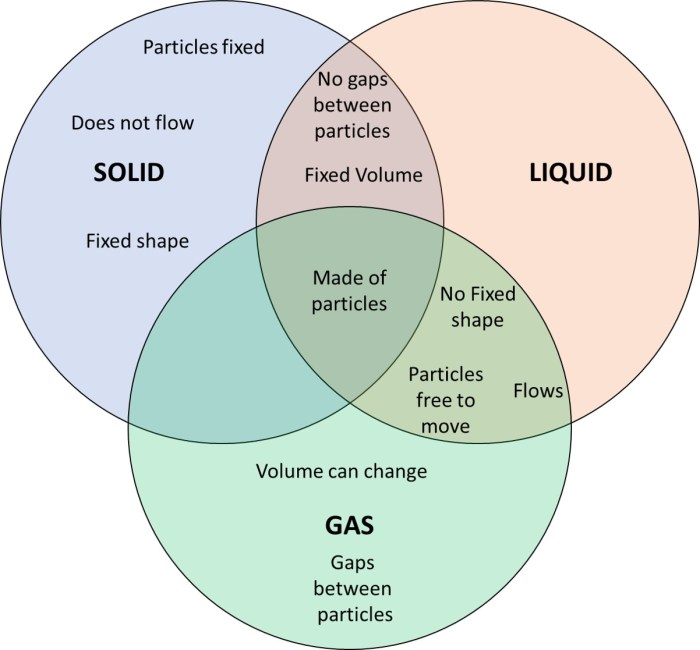
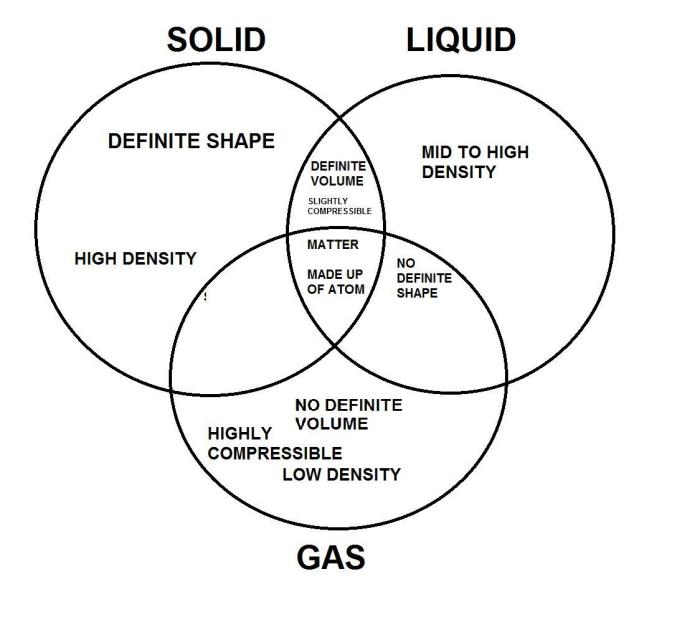
A Venn diagram is a graphical representation that shows the similarities and differences between two or more sets. In the case of solids, liquids, and gases, a Venn diagram can help us to visualize the properties that are shared by these states of matter, as well as the properties that are unique to each state.
Overlapping Regions
The overlapping regions of the Venn diagram represent the properties that are shared by multiple states of matter. For example, all three states of matter have mass and volume. Solids and liquids both have a definite shape, while liquids and gases both flow.
Gases and solids both can be compressed.
Implications
The Venn diagram can help us to understand the behavior of matter by showing us how the properties of solids, liquids, and gases change as the temperature and pressure change. For example, the diagram shows us that solids become liquids when they are heated, and liquids become gases when they are heated further.
The solid-liquid-gas Venn diagram is a visual representation of the three states of matter. It can be used to show the properties of each state and how they change as matter transitions from one state to another. Dept 56 The Old Globe Theater is a miniature replica of the original Globe Theatre in London.
It is made of porcelain and is hand-painted. The solid-liquid-gas Venn diagram can be used to show the different materials used in the construction of the theater.
The diagram also shows us that gases can be compressed into liquids, and liquids can be compressed into solids.
Applications of State Changes
Phase transitions have numerous practical applications in industry, science, and everyday life. Understanding these changes is crucial for various fields, including chemistry, physics, engineering, and materials science.
In Industry
- Food processing:Phase transitions are used in freezing, canning, and drying food to preserve it and extend its shelf life.
- Pharmaceuticals:Phase transitions are involved in the production and purification of drugs, as well as in drug delivery systems.
- Chemical engineering:Phase transitions are utilized in processes such as distillation, crystallization, and extraction.
In Science
- Materials science:Understanding phase transitions is essential for developing new materials with desired properties, such as superconductivity, shape memory, and high strength.
- Earth sciences:Phase transitions play a role in geological processes, such as the formation of rocks and the movement of tectonic plates.
- Climate science:Phase transitions between water, ice, and vapor are crucial for understanding the Earth’s climate system.
In Everyday Life
- Refrigeration:Phase transitions are used in refrigerators and air conditioners to remove heat from food and rooms.
- Cooking:Phase transitions are involved in cooking processes, such as boiling, frying, and baking.
- Cleaning:Phase transitions are utilized in cleaning processes, such as washing clothes and dishes.
Factors Affecting State Changes
The transformation of matter from one state to another is influenced by several key factors. Understanding these factors allows us to manipulate and predict the behavior of substances.
The three main factors affecting state changes are temperature, pressure, and the presence of impurities.
Temperature
Temperature plays a crucial role in phase transitions. Generally, increasing the temperature of a substance causes it to melt, boil, or sublime. For example, when ice is heated, it melts into liquid water at 0 degrees Celsius. Similarly, when water is heated to 100 degrees Celsius at sea level, it boils and transforms into water vapor.
Pressure
Pressure also affects the melting and boiling points of substances. Increasing pressure typically raises the melting point and lowers the boiling point. This is why water boils at a higher temperature in a pressure cooker compared to an open pot.
Impurities
The presence of impurities can affect the melting point and boiling point of a substance. Impurities can lower the melting point and raise the boiling point. For example, adding salt to water lowers its freezing point, which is why salt is used to melt ice on roads during winter.
Exceptions to the Rule
Phase transitions typically follow well-established patterns, but some substances exhibit exceptional behavior. These exceptions provide valuable insights into the complex nature of matter and challenge our understanding of its properties.
Supercritical Fluids, Solid liquid gas venn diagram
Supercritical fluids are substances that exist above their critical temperature and pressure, where the distinction between liquid and gas phases vanishes. They possess unique properties that combine the characteristics of both liquids and gases. Supercritical fluids are highly compressible like gases but have densities comparable to liquids, making them excellent solvents for various industrial applications.
Amorphous Solids
Amorphous solids, also known as glasses, lack the long-range order of crystalline solids. Instead, their atoms or molecules are arranged in a disordered, non-repeating pattern. This unique structure gives amorphous solids properties distinct from both liquids and crystalline solids. They are typically transparent, brittle, and have a high viscosity, making them useful in applications such as windows, containers, and optical fibers.
Reasons for Exceptions
The exceptional behavior of these substances can be attributed to various factors, including:
-
-*Molecular Structure
The molecular structure and intermolecular forces play a crucial role in determining the phase behavior of substances. Supercritical fluids, for instance, have molecules with weak intermolecular forces, allowing them to exist in a continuous phase above their critical point.
-*Thermodynamic Conditions
The temperature and pressure conditions can influence the phase behavior of substances. Amorphous solids are formed when a liquid is cooled rapidly, preventing the atoms or molecules from arranging themselves into a crystalline structure.
Historical Perspectives
Our understanding of the states of matter has evolved over centuries through experimental observations and theoretical advancements.
In the 17th century, Robert Boyle’s experiments on gases led to Boyle’s Law, which describes the inverse relationship between the volume and pressure of a gas at constant temperature.
Contributions of Charles and Dalton
In the 18th century, Jacques Charles and Joseph Gay-Lussac discovered that gases expand at a constant rate with increasing temperature, leading to Charles’s Law and Gay-Lussac’s Law.
John Dalton’s atomic theory, proposed in the early 19th century, explained the behavior of gases in terms of tiny, indivisible particles in constant motion.
Top FAQs
What is the purpose of a solid liquid gas venn diagram?
A solid liquid gas venn diagram is a visual representation of the similarities and differences between the three states of matter. It helps to illustrate the properties that are shared by multiple states, as well as the unique characteristics of each state.
How can I use a solid liquid gas venn diagram in my science class?
A solid liquid gas venn diagram can be used in a science class to teach students about the different states of matter and their properties. It can also be used to help students understand phase transitions and the factors that affect them.
What are some examples of phase transitions?
Melting, freezing, vaporization, and condensation are all examples of phase transitions.